Echo Acoustic
MASS SPECTROMETRY
Accelerating high throughput screening and biochemical assay development
Introduction to Ultra-High Throughput Acoustic Mass Spectrometry

Applying Acoustic Mass Spectrometry Technology to Biochemical Screening and Beyond
Dr. Jonathan Wingfield , Principal Scientist at AstraZeneca, discusses the development of acoustic mass spectrometry technology for high-throughput screening. Through the combination of acoustic technology from Labcyte and mass spectrometry systems from Waters, acoustic mass spectrometry provides rapid sample processing and analysis of a wide range of molecular targets, saving crucial time and reagents costs needed to screen millions of compounds within AstraZeneca’s small molecule and discovery programs.
Upside of Liquid Chromatography-MS
Liquid chromatography–mass spectrometry (LC-MS) is a fantastically capable analytical tool. With the high resolution of new accurate-mass systems, the qualitative and quantitative performance is unrivaled. The technique’s label-free nature simplifies assay development, reducing both time and cost. LC-MS delivers high content data which not only identifies compounds from complex matrices, but can also provide molecular fragmentation data fundamental for structural elucidation.
Downside of LC-MS
Unfortunately, LC-MS suffers when it comes to speed. HPLC/UPLC-MS’s ability to deliver results at a rate of between 1–10 minutes per sample may be perfectly acceptable in some cases. However, if you need to screen a library of a million or more compounds, the pace of LC-MS makes it unusable. At 0.5 mL/min, and 5 minutes/sample, screening a million-compound library would consume 2,500 liters of mobile phase.
At about 10 seconds per sample, Automated Solid Phase Extraction-MS, a slimmed down version of LC-MS, is much faster. However, it is still too slow for prosecuting large library screens. High throughput MALDI-MS is very fast, but comes with the overhead of complex sample preparation and the risk of cross-contamination.
Enter Labcyte
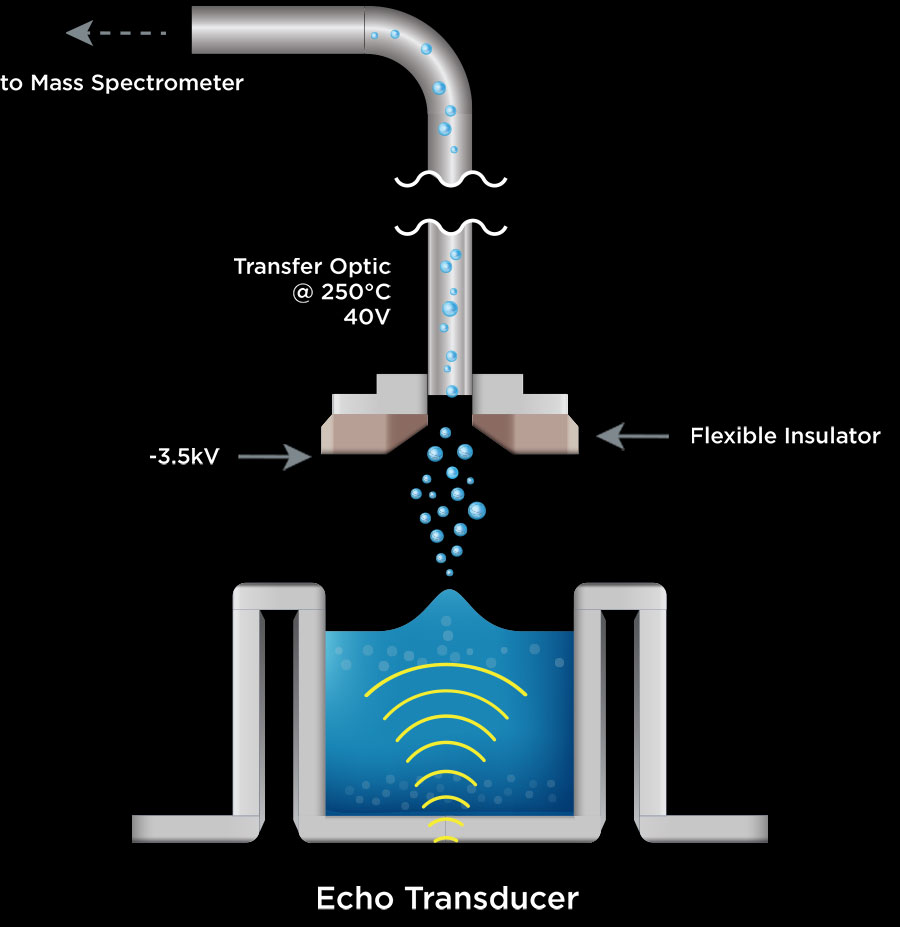
Over the past 15 years, Labcyte has demonstrated a consistent history of innovation in the use of acoustic energy to transfer liquid samples without making actual contact with the sample. A gentle burst of ultrasonic energy creates a mound on the liquid surface in a source well and then ejects a 2.5 nL droplet of sample. Thanks to surface tension, this droplet can be captured by an inverted well located over the source well. This occurs very quickly, at a rate of up to 500 Hz. This fast, contamination- and carryover-free liquid handling technology has become a standard tool in many areas of life science research, including cancer, genomics and pharmaceutical drug discovery.

Matching Acoustic Delivery to Mass Spectrometry
Research into droplet formation led to the ability to generate a mist of nano-droplets similar to those created in a mass spectrometer’s electrospray ionization source. Working in collaboration with the AstraZeneca and Waters Inc., a prototype of a new ionization source has been designed to demonstrate this technology’s potential.
We refer to this technology as Acoustic Mist Ionization, or AMI. Combined with a Waters Xevo G2-XS, the Acoustic-MS system can analyze a 384-well microplate at better than 3 wells per second. Unlike LC-MS, there is no consumption of mobile phase, saving huge amounts of solvent.

For large sample sets, data is collected, compiled, and the results viewed as a heat-map, displaying the presence/absence of a target compound. The mass spectrum for each well can be subsequently inspected.
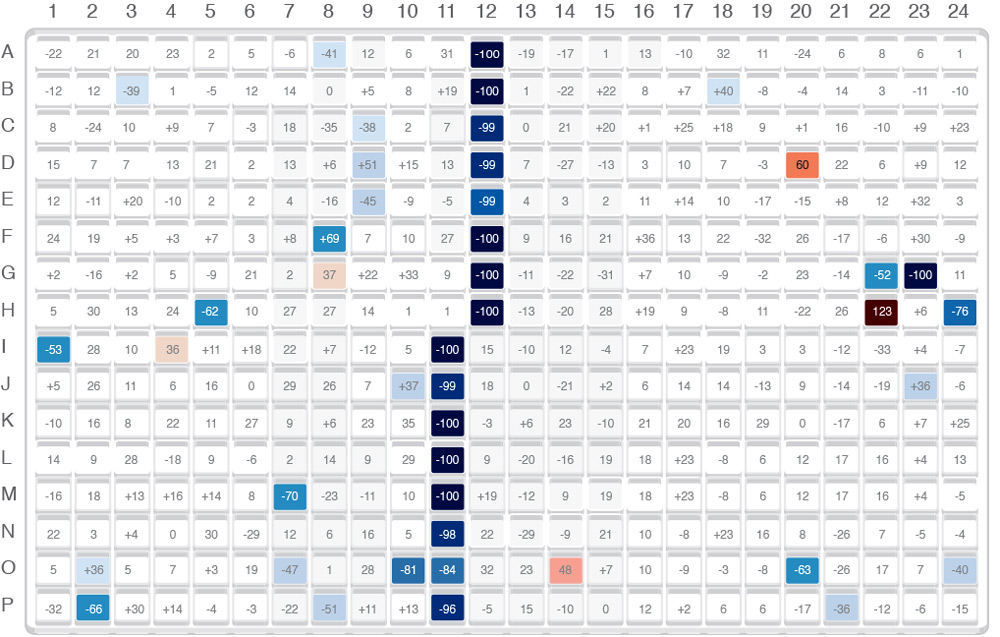
Using Prototype Acoustic-MS Systems
In tests using real samples, Acoustic-MS has shown itself to be a reliable technology with unparalleled speed. In an early trial, the system enabled AstraZeneca to save 95% of the cost to develop the assay. It also reduced the development time from an estimated 6 months to 2 weeks, and screened 230,000 compounds (600 plates) in 10 working days. A subsequent screen of a 2.5 million compound library against a different target was completed in 40 days, with a demonstrated capability to process over 100,000 samples per workday. Because of the high-quality MS data, there were fewer false positives needing further investigation.
Image courtesy of AstraZeneca
Big Potential for Acoustic-MS
The emerging technology of Acoustic-MS offers the promise of ultra-high throughput (100,000 samples per day), high quality MS data, and label-free assay development with low sample consumption. We anticipate it will soon become a commercially available system with tremendous power to overcome the challenges of high throughput biochemical analysis.

